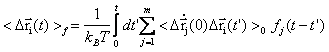關於我們近五年的分子設計研究:
https://drive.google.com/file/
(In this molecular biophysics and bioinformatics lab, we use a spectrum of physical models to study protein/RNA conformational changes; the dynamics features are used to understand biomolecular functions, predict enzyme active sites, Protein-DNA binding sites and protein-protein binding interfaces etc. Normal mode analysis, time-dependent linear response theory and molecular dynamics simulations are used to refine X-ray/NMR-determined structure complexes, understand intra-/intermolecular signal communication, design antimicrobial peptides, explore protease degradation mechanism, and understand ribosomal helicase activities in unwinding secondary structures of mRNA. We also develop new Bioinformatics tools such as fast comparison tools for long text strings and chromosome identification algorithms using the reads derived from Next Generation Sequencing)
Despite of diversity of lives, there are common rules governing them.
Linear information carried by genes is translated into three-dimensional functional units that assume various forms and regulate variety of functions. Nonetheless, they all serve a common purpose – to keep lives alive, under the given selection pressure.
A consensus among these functional units, namely the protein structures, is the physical forces that govern interactions of their constituent atoms. At the molecular level, the molecules fold, interact and change their configurations following fundamental physical rules. The molecular functions and therefore the organism survival (or evolution) should also be refrained by these rules. Biodiversity cannot be more diverse than physics would allow.
In this lab, we use physical models and computation simulations to study protein conformational changes (in the context of equilibrium dynamics), and interactions. We are here to understand why certain structures dictate certain functions, and also why certain functions require certain structures and dynamics to perform.
We would like to find the rules, the consensus underlining things that are seemingly different.
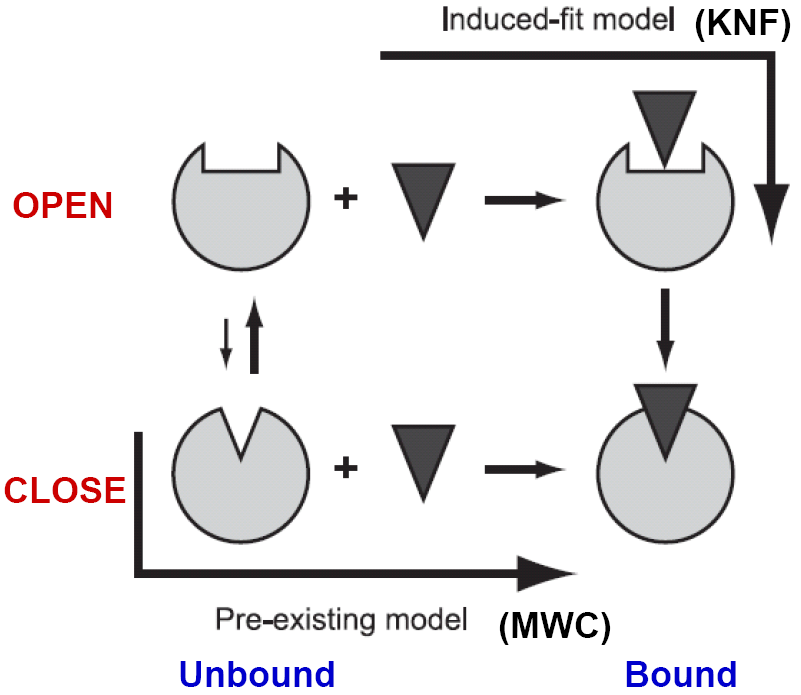
Conformational changes of biomolecules occur to accommodate substrate or to facilitate interactions with their binding partners form the simplest dynamic picture of life in the molecular level. KNF (induce-fit) and MWC (pre-existing equilibrium) models were suggested decades ago to explain the allosteric phenomenon of hemoglobin. However, the physical origin(s) of the two models has not been understood in enough depth at the molecular level so as to properly explain the observed conformational changes.
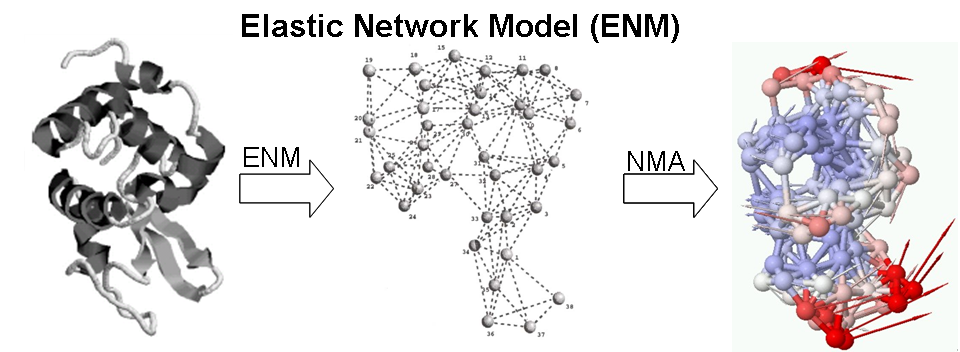
At physiological temperature and pressure, biomolecules have their intrinsic motions about the mean. A pre-existing state, deviating from the mean at elevated energy, could favor a binding event, either by a ligand or other protein partners. On the other hand, an incoming ligand, landing in the open form of the receptor, could induce a closure of protein domains resulting in a binding event, via which one of the two seemingly different pathways for free proteins to migrate into bound complexes highlights a constant debate for decades. We use elastic network models (ENMs) and linear response theory (LRT) to study the nature of these two pathways.
Mechanochemical Activity of Enzymes
To understand the coupling between collective dynamics and catalytic activity of the enzyme, a systematic analysis of 98 non-homologous enzymes of different EC classes were analyzed using GNM, fluctuation patterns near the known catalytic residues (experimentally known) are studied. In more than 70% of the examined enzymes, the hinge minima predicted by the GNM are found to be co-localized with the catalytic sites. Low rotational mobility (<7%) was observed for the catalytic residues consistent with the functional necessity of enzymes to achieve precise mechanochemical activities . These studies agree with previous studies that hinge flexibility is an important mechanism in determining protein conformation and facilitating ligand binding , in mediating allosteric effects or fine-tuning enzyme functions.
Chemistry-free model for Characterizing/Predicting Key Residues that Control Enzymatic Functions (with Drs Ivet Bahar and Akio Kitao)
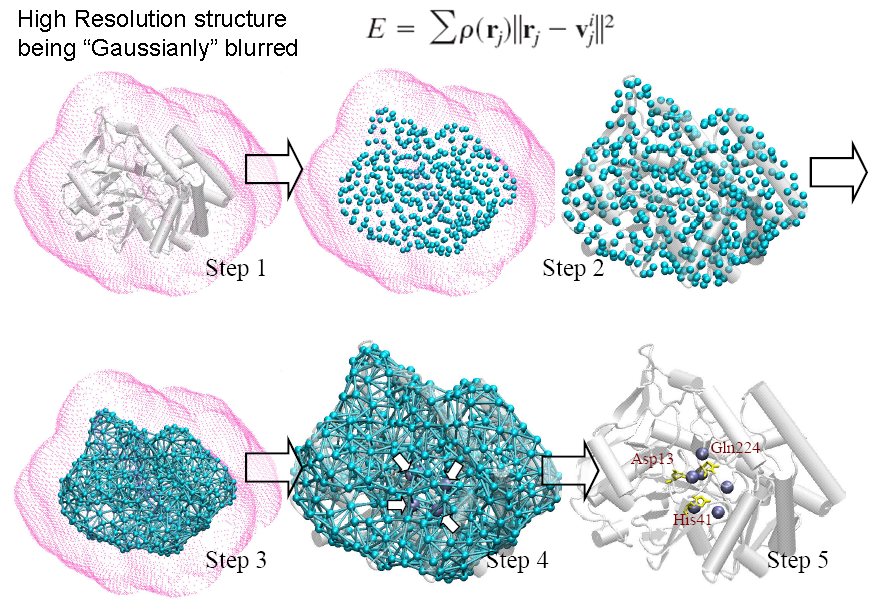
A de novo algorithm, COnformational-Mobility-based Prediction of enzyme ACTive sites (COMPACT) , was developed to further test the idea that ‘enzyme active sites co-localized with the dynamic hinge centers’. COMPACT, a protein active site predictor based on coarse-grained structure (shape of the molecule) and dynamic information, has scored a selectivity rate of 0.82 and a moderate to low specificity rate 0.37 over 18 enzymes in different enzyme classes. COMPACT basically narrows down potential candidates of catalytic residues, hinge minima, predicted from GNM, to spatially clustered ones with relatively high solvent accessibilities for its position in the protein. This allows the prediction for biomolecules’ functional sites without even knowing the amino-acid sequence and atomic coordinates. We repeated a similar procedure on X-ray electron density maps of enzymes and were able to locate the position of the functional sites within the electron cloud with a sensitivity and specificity of 0.73 and 0.33 respectively. The restrained dynamics can be of an important active site design properties and the prominent geometrical shape of enzyme is highly related to its active site location. The online implementation of this prediction algorithm can be found in oGNM and Dynomics.
Time-dependent Linear Response Theory to studying primary responses of myoglobin (with Drs Nobuhiro Gō and Akio Kitao)
The primary response of proteins occurs at femto- to picosecond range is understood as protein response to force perturbations. The extent of response at a given atom i can be expressed as the sum of component ij of fluctuation variance multiplying force fj applied on atom j [23].
In the past year, we have formulated a time-dependent response function
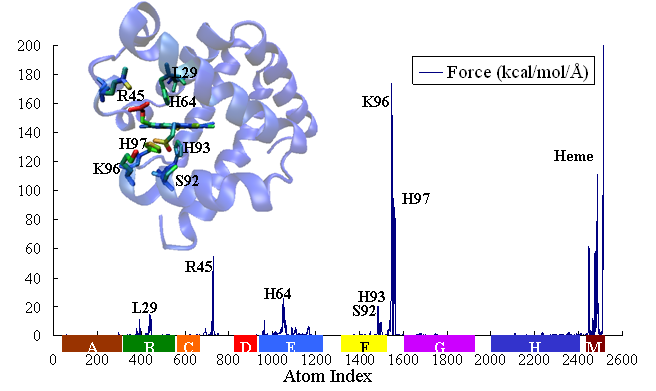
,within which the time-dependent covariance matrix (or so derived matrices) can be expressed in the normal mode space with solvent friction taken into account [22] (friction is obtained from velocity time correlation function, calculated from MD trajectory). Proteins, projected into each normal mode, as a single oscillator subject to solvent friction and random forces, can be traced to its time-dependent conformational changes upon external or internal perturbations (e.g photodissociation to release otherwise bound monoxide ligand). We study the time course of structural changes between deoxy- and carbonmonoxy myoglobin (CO-Mb) upon multi- or single point (at Fe of heme) force perturbations and compare the results with observables of UV resonance raman (UVRR) spectrum upon photodissociation of CO. A qualitative and quantitative agreement has been obtained for the relaxation and primary-response time constants at respectively 10s picoseconds and 100s femtoseconds to a few picoseconds.
Prediction of catalytic rate of enzyme mutants and mechanical reasons for some evolutionarily conserved residues – theory and experiment (with Dr Shakhnovich)
Allosteric regulation in enzyme activity can be seen as the ability of proteins to visit many conformational states. Mechanical energy is required for protein to take from one state to the other and the binding/hydrolysis of a cognate substrate can provide such energy. We hypothesize that there are some key mechanical residues (KMR), evolutionarily conserved, which could perturb the energy dramatically upon mutation, and therefore possibly jeopardize enzyme activity from places remote to the catalytic center. People have shown experimentally that single mutation G121V of βF-βG loop, which is more than 19Å away from the closest atom of DHF at the active site, reduces the rate of hydride transfer in DHFR by 163 fold. Also, double mutation M42F-G121A impairs enzyme activity by 21 times more than individual effects combined would do (i.e. highly non-additive) when the two residues are 20Å away from each other. We would first apply more comprehensive, published approaches that are to use classic + quantum mechanics simulations to predict mutant activities. Then, we will attempt to develop purely classic-mechanics-based simple models to tackle the same issue. Shall this be successful, we will then apply the models to other enzymatic systems to verify our first-principal-based understandings of enzyme catalytic mechanism. We will also have a wet lab to verify our predictions in a uniformed, controlled environment. This theory-joint-experiment project will first identify a few KMR and then theoretically predict by how much the catalytic rate would reduce/increase if the possibility for DHFR to visit a catalysis-prompted bound state is impaired/boosted by a given mutation.
To understand how chemical modifications (mutation) would affect protein stability and function is essential to elucidate the relation between organismal genotype (determining molecular physics) and phenotype (determining population dynamics of the organism). See an introductory lecture here.